Worksheet Naming Ionic Compounds Worksheet Answer Key —
Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.

Formulas And Nomenclature Binary Ionic Compounds Worksheet Answers
compounds Worksheet-Answer Key. Name Date. Nomenclature is a system of naming. This worksheet presents a widely used system of nomenclature for ionic compounds. There are two types of metal cations with different naming conventions discussed separately. Part A: Fixed charge (single charge) cations; Part B: Variable charge (multiple charge) cations

33 Naming Ionic Compounds Worksheet Answer Key support worksheet
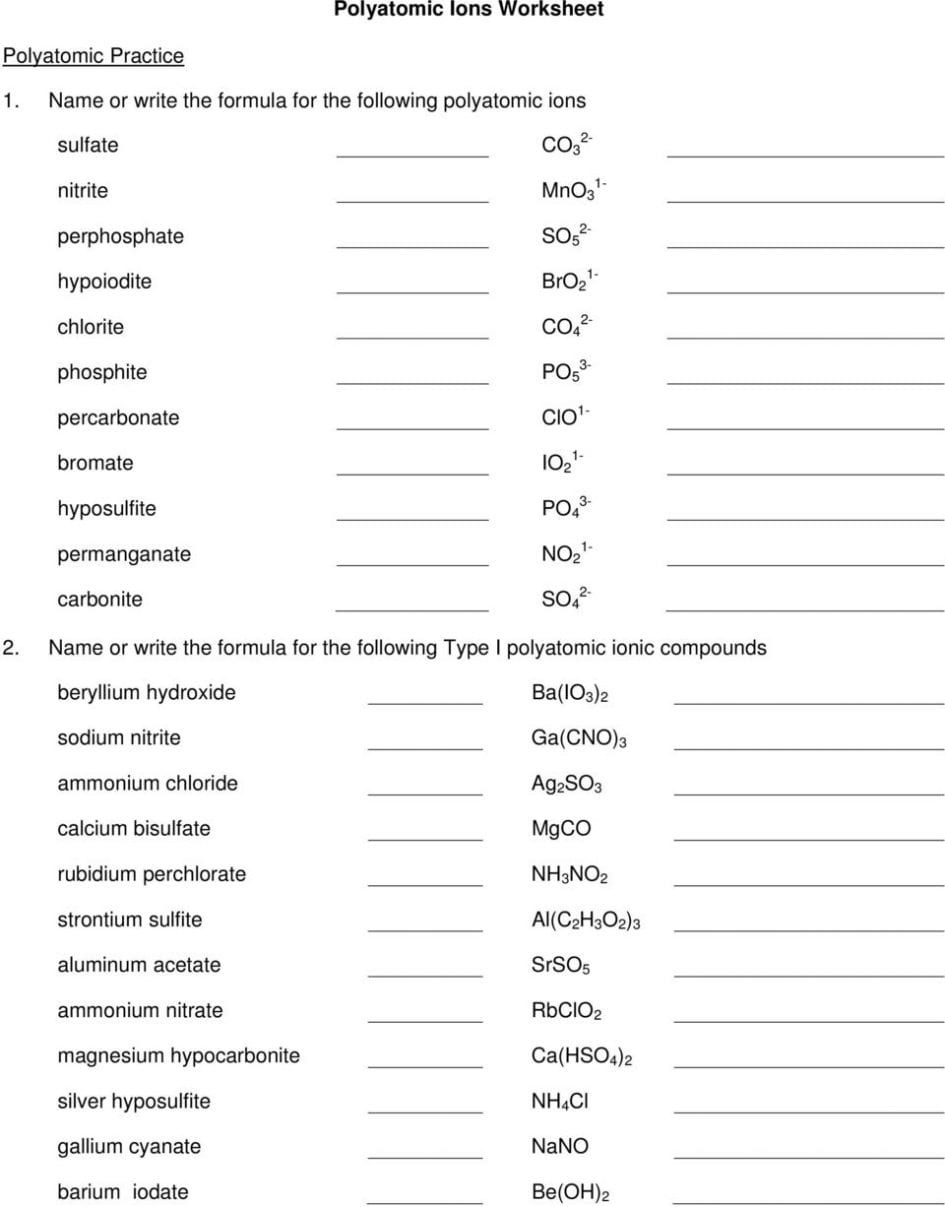
Ternary Ionic Compounds For each combination of ions, fill in the chart below: cation symbol anion symbol # cations needed # anions needed formula compound name calcium + nitrite barium + sulfate silver + acetate nickel(II) + phosphate sodium + carbonate lithium + bicarbonate ammonium + phosphate beryllium + hypochlorite calcium + hydroxide
Naming Covalent Compounds Worksheet Quizlet buzzinspire
Ionic Compound Naming Worksheet. Practice naming ionic compounds. This worksheet tests your ability to give names and formulas. The worksheet and answer key are in PDF format for easy downloading and printing. [PDF Worksheet] References. Fernelius, W. Conard (November 1982). "Numbers in chemical names".

15 Best Images of Practice Naming Ionic Compounds Worksheet Naming
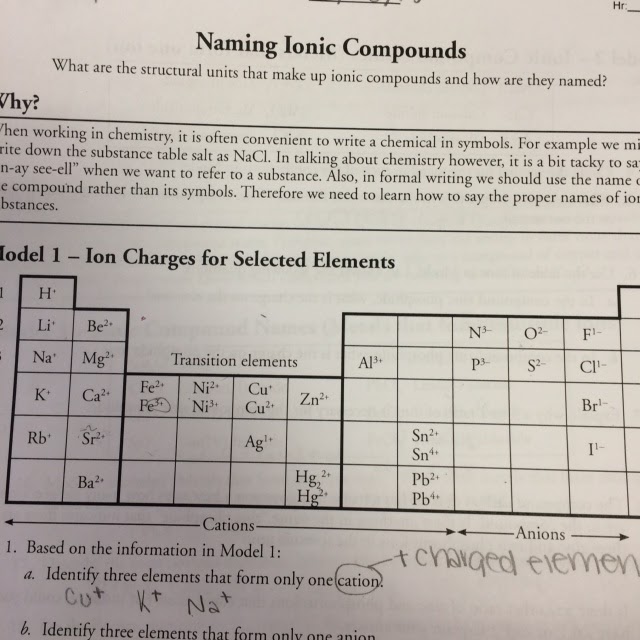
The key is usually found in the elements that form the first two columns of the periodic table. These are the alkali and alkaline earth metals. They are very commonly found in ionic compounds, and they are reliably found as cations in those compounds. The alkali metals are always found as 1+ or monovalent cations, such as Na +, K +, or Li.

binary practice worksheet
Ionic compounds are held together by ionic bonds. They are named using the cation name first, followed by the anion name, excluding the word "ion.". For example, sodium ion (Na +) and chloride ion (Cl -) form the compound sodium chloride. Its formula is NaCl, which is electrically neutral because sodium ion is +1 and chloride ion is -1.

8 Best Images of Organic Chemistry Review Worksheets Chemistry
Binary Ionic Compounds ANSWER KEY. Directions: First quickly scan the worksheet and circle any metals (as a symbol or as a name) that is a transition metal. Then either give the same or chemical formula as necessary for each problem below. Remember that transition metals can have multiple oxidation states, so you are required

Writing And Naming Binary Ionic Compounds Worksheet Answer Key
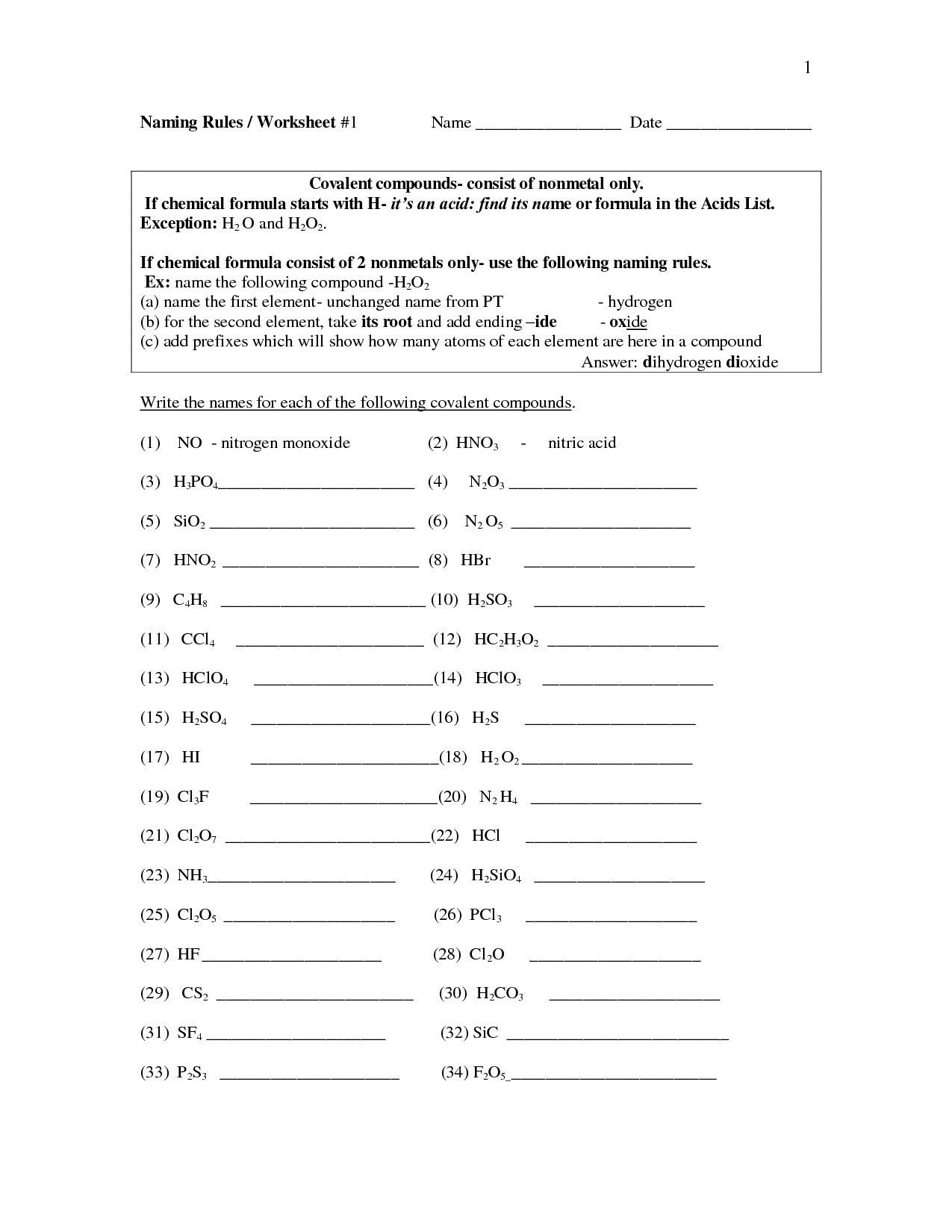
Name the following compounds: (a) NaF (b) Rb 2 O (c) BCl 3 (d) H 2 Se (e) P 4 O 6 (f) ICl 3. Answer a. sodium fluoride. Answer b. Rubidium oxide. Answer c. boron trichloride. Answer d. hydrogen selenide. Answer e. tetraphosphorous hexaoxide (if you are googling the answers to your homework, google may disagree with you.
Naming Ionic Compounds Worksheet 1 Answer Key Worksheets For All
If the formula begins with a metal and ends with two or three nonmetals then it must be a polyatomic ion in the formula. Steps for naming: 1. Name the metal with its full name. 2. Identify the polyatomic ion at the end of the formula, use its name. naming examples: Ca(NO3)2 KNO3 Ba(OH)2 Li2CO3 Al2(SO4)3 NH4ClO3.

naming ionic compounds worksheet 1 answer key
Naming Ionic Compounds Practice Worksheet Name the following ionic compounds: 1) NH 4 Cl _____ 2) Fe(NO 3) 3. Answers - Naming Chemical Compounds 1) NaBr sodium bromide 2) Ca(C 2 H 3 O 2) 2 calcium acetate 3) P 2 O 5 diphosphorus pentoxide 4) Ti(SO 4) 2 titanium(IV) sulfate

Naming Ionic Compounds Worksheet 2 Answer Key
Memorize the ionic charges for Ag+ and Zn2+. 3. All other transition metals have multiple charges. Use a roman numeral to indicate the cation charge, which can be figured out from the given information. Exercise 4. Provide the name for each compound. Nomenclature for ionic compounds Worksheet-Answer Key Dr. Scott Beaver Page 5 of 5

Ionic Compounds Worksheet Answers Printable Word Searches
Write the formulas of the following acids and bases: 99) hydrobromic acid HBr 100)hydrofluoric acid HF 101) carbonic acid H2CO3 102) lithium hydroxide LiOH 103) nitrous acid HNO2 104) cobalt (II) hydroxide Co(OH)2 105) sulfuric acid H2SO4 106) beryllium hydroxide Be(OH)2.

Naming Ionic Compounds Worksheet Answer Key Pdf › Athens Mutual Student
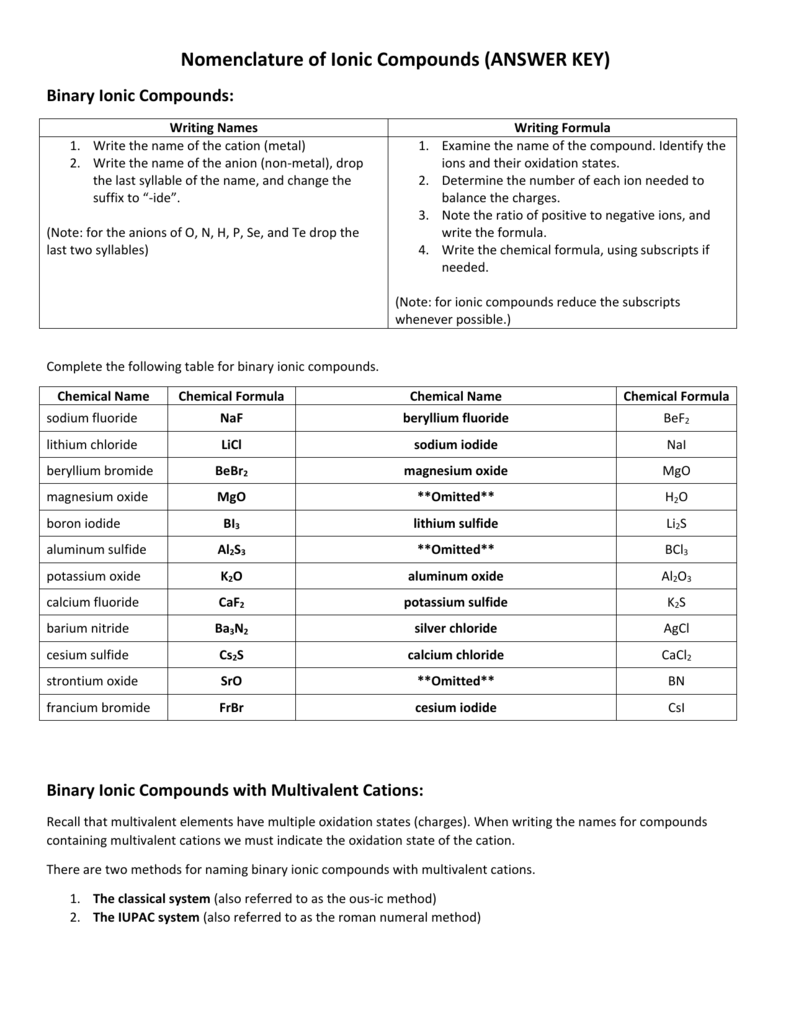
Name the following ionic compounds using what you learned from Model 2. Li 2 O Lithium oxide MgF 2 Magnesium fluoride. Al 2 S 3 Aluminum sulfide K 3 N Potassium nitride 13. Provide the chemical formula for each of the following ionic compounds. Helpful subscripts to cut-and-paste electronically: ₂ ₃ ₄. Barium chloride BaCl 2 Magnesium.
Naming Ionic Compounds Worksheets Answer Key Pogil
Naming Chemical Compounds - Answers Name the following ionic compounds: 1) NaBr sodium bromide 2) CaO calcium oxide 3) Li 2 S lithium sulfide 4) MgBr 2 magnesium bromide 5) Be(OH) 2 beryllium hydroxide Write the formulas for the following ionic compounds: 6) potassium iodide KI 7) magnesium oxide MgO 8) aluminum chloride AlCl 3
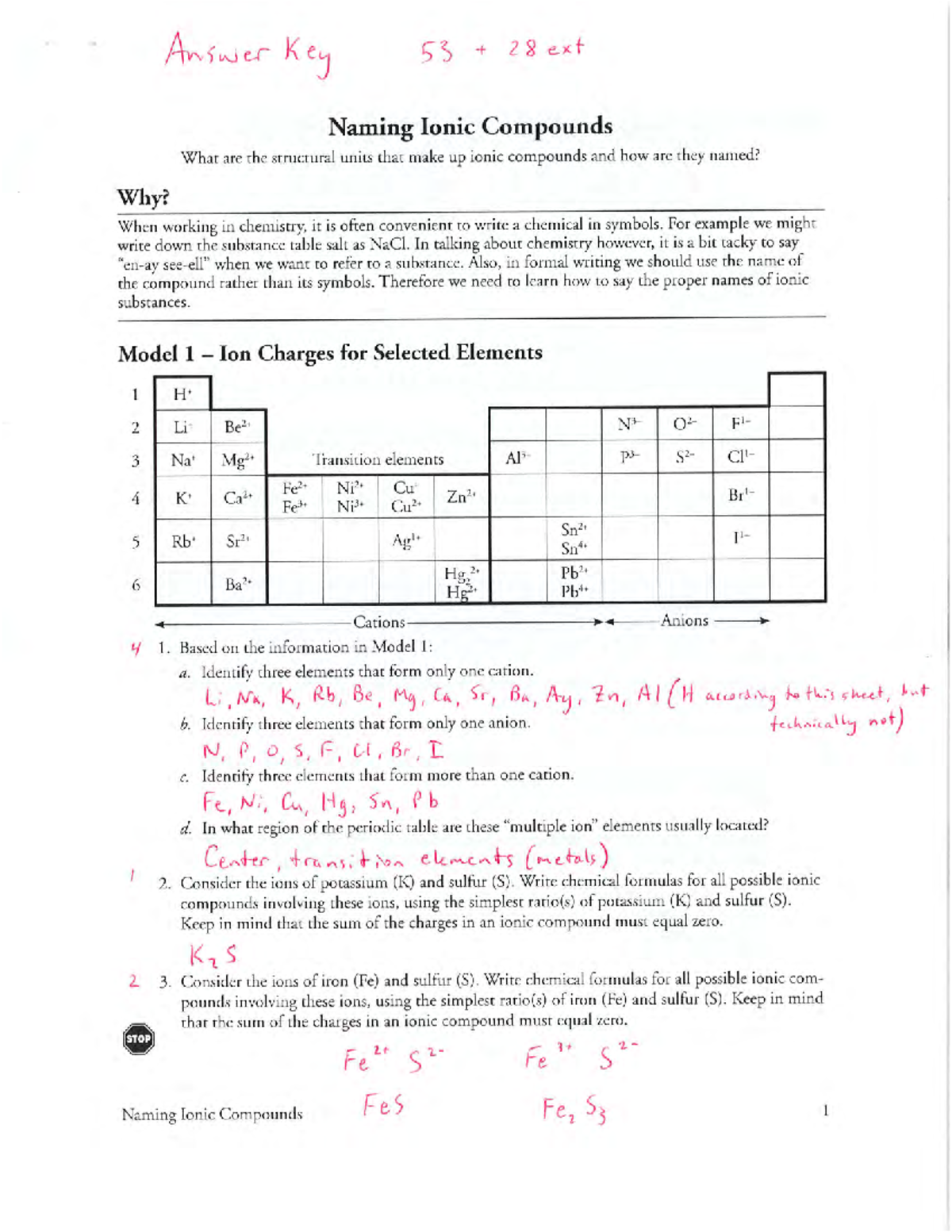
15 Naming Ionic Compounds answer key CHEM 1001 Studocu
Ionic Naming Practice Problems - Solutions Name the following ionic compounds: 1) NaBr sodium bromide 2) Sc(OH)3 scandium hydroxide 3) V2(SO4)3 vanadium (III) sulfate 4) NH4F ammonium fluoride 5) CaCO3 calcium carbonate 6) NiPO4 nickel (III) phosphate 7) Li2SO3 lithium sulfite 8) Zn3P2 zinc phosphide 9) Sr(C2H3O2)2 trontium acetates.

Forming And Naming Binary Ionic Compounds Worksheet Answer Key
Ionic Compounds: Names and Formulas Worksheet 1. Write the formulas for the following compounds. (a) magnesium oxide MgO (bl sodium fluoride (cl aluminum nitride (d) potassium sulfide (e) lithium iodide (fl calcium bromide (g) beryllium oxide (11) (h) nickel,., chloride (fl magnesium nitride (j) aluminum sulfide 2. Write the names for the.